From Yttria to Promethium: The 200-Year Search for Rare Earth Elements
Table of Contents
Rare earth elements are essential to modern technology, powering everything from smartphones to electric vehicles, but their discovery was anything but straightforward.
This article traces the centuries-long journey of chemists who, starting with mysterious minerals found in Swedish mines, unraveled the identities of 17 elusive elements.
Through persistent experimentation and breakthroughs in spectral analysis, these once-misunderstood “rare earths” transformed from scientific curiosities into indispensable resources, showing that real progress is built on patience and generations of discovery.
For more on the importance of understanding scientific history, see my earlier post, Looking Back to Move Forward: The Power of Scientific History, where I explore why awareness of past discoveries can deepen our perspective on current science.
Rare earth elements don’t get much attention in daily conversation, but they’re the hidden heroes of modern technology. Without them, your smartphone wouldn’t light up, electric cars wouldn’t run, and wind turbines wouldn’t spin efficiently. Rare earths began to be discovered over 200 years ago, starting with yttrium in 1794, though their unique technological potential wasn’t fully recognized until the mid-20th century.1
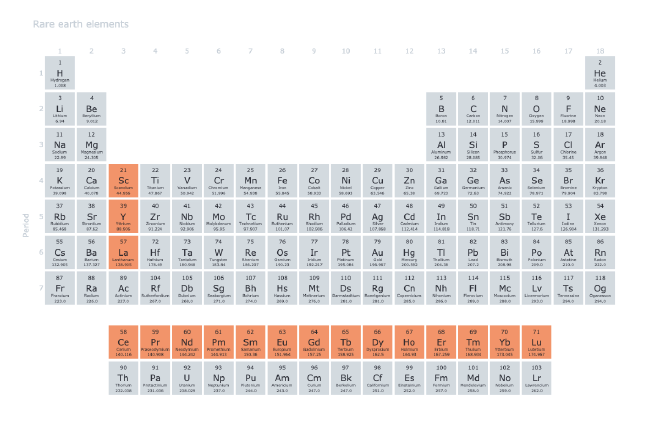
The rare earth family includes 17 elements: scandium, yttrium, and the 15 lanthanides, which sit between barium and hafnium on the periodic table. The story begins with yttrium, discovered by Finnish chemist Johan Gadolin in the late 1700s. But this was just the first piece of a much larger puzzle.2 3
At the time, the scientific world was buzzing with new discoveries. Researchers were isolating elements left and right, but rare earths proved particularly tricky to understand. When scientists extracted them from minerals like yttria and ceria, these elements behaved in ways that didn’t fit any known patterns. This mystery would take generations to solve—and would eventually transform technology as we know it.
The Misleading Name: Why Are They Called “Rare” Earth Elements? #
After discovering these mysterious elements in Swedish mines, early scientists made an assumption that would stick for centuries—they called them “rare earths.” However, like many scientific terms coined in the heat of discovery, this name reflects more about what early chemists thought than what we understand today.
When researchers first isolated these elements in the late 1700s, they encountered them as oxides—substances chemists then referred to as “earths” (from the French terre and German Erde).4 These “earths” appeared in minerals like yttria and ceria, seemingly found only near the village of Ytterby, Sweden. Finding them in this one location led scientists to believe they were scarce, hence “rare.”5 6
The irony? Rare earth elements aren’t actually rare at all. They are more abundant in Earth’s crust than familiar elements like mercury and cadmium. The challenge lies in how they occur in nature—scattered in tiny amounts across many minerals rather than concentrated in rich deposits. It’s like having a thousand euros in cents scattered across a beach rather than a single ten-euro bill in your pocket.
What really made rare earths difficult to work with wasn’t their scarcity but their similarity to one another. These elements are chemical cousins with nearly identical atomic structures, making it extremely challenging to separate them. Imagine sorting a mix of dark blue and navy blue marbles by color, and you’ll get the idea. Scientists needed sophisticated techniques to distinguish and isolate each element.
This chemical similarity also explains why the original sources—yttria and ceria—weren’t unique after all. Scientists later found the same elements in monazite, a mineral-rich sand that would become crucial for rare earth production. What began as a seemingly unique curiosity from a Swedish village ultimately revealed itself to be a family of elements dispersed throughout Earth’s crust.
The First Discoveries: When Rare Earths Challenged Chemistry #
The late 18th and early 19th centuries were transformative for chemistry. Scientists like Boyle, Lavoisier, and Dalton were fundamentally reshaping our understanding of matter, establishing that elements were the simplest substances—materials that couldn’t be broken down further by chemical means. This breakthrough led to a cascade of discoveries, with scientists identifying nearly 70 of the 90 naturally occurring elements by the mid-19th century.4
Among these discoveries, however, emerged an unusual set of elements: the rare earths. In 1787, researchers identified two uncommon minerals, yttria and ceria, containing elements unlike any previously known. These elements posed a unique challenge: their chemical properties were so similar that scientists struggled for decades to separate and identify each one individually. This painstaking process of discovery continued well into the 20th century, finally culminating with the isolation of promethium in 1947.
Even the pioneering chemists Dmitri Mendeleev and Lothar Meyer, who developed the first periodic tables, were stumped by the rare earth elements. These elements didn’t fit neatly into their structured systems. Only with the advances in atomic theory during the 20th century did scientists begin to understand what made rare earths so distinctive—and why they had proven so difficult to classify.
From Black Stone to Breakthrough: The Discovery of Yttria #
The story of how rare earths puzzled the scientific world begins with a black stone and a curious army officer. In 1787, Carl Axel Arrhenius, a Swedish officer and geologist, discovered an unusual black mineral in the village of Ytterby.7 Though he simply called it “black stone,” this seemingly unremarkable specimen would spark centuries of scientific investigation.
The stone’s journey through scientific circles began gradually. Swedish chemist Bengt Reinhold Geijer published the first report on its unusual properties in 1788, but the real breakthrough came in 1794 when Finnish chemist Johan Gadolin conducted a thorough analysis. His findings were striking: nearly 38% of the stone consisted of an unknown “earth” (now recognized as an oxide), along with iron and silicate. Gadolin realized he had uncovered something significant, though its full nature remained uncertain.
In 1795, Swedish chemist Anders Ekeberg confirmed Gadolin’s findings and gave the oxide the name “yttria” in honor of its place of origin, Ytterby. Early chemists, including Ekeberg, believed these “earths” were elements themselves—a misconception that persisted until 1808, when Sir Humphry Davy’s electrolysis experiments suggested yttria was a compound. The metallic element within yttria would later be isolated and named yttrium.
The black stone continued to reveal secrets. In 1802, Ekeberg identified yttria in another mineral, yttrotantalite, which led him to discover tantalum. Meanwhile, the initial findings gained further validation when German chemist Martin Heinrich Klaproth and French chemist Louis Nicolas Vauquelin independently confirmed Gadolin’s work. In recognition of Gadolin’s pioneering role, Klaproth named the original mineral “gadolinite”—securing Gadolin’s place in rare earth history.
Ceria: A Tale of Double Discovery #
While Gadolin and others were unraveling the mysteries of yttria, another rare earth story was emerging. A different Swedish mineral, cerite, had puzzled chemists since the mid-1700s. Scientists suspected it contained an unknown “earth,” but proving this would take decades—and lead to a remarkable scientific coincidence.
In 1803, two independent teams solved the puzzle almost simultaneously. On one side were Swedish chemists Jöns Jacob Berzelius and Wilhelm Hisinger; on the other was German chemist Martin Heinrich Klaproth—the same scientist who had confirmed Gadolin’s work on yttria. In an extraordinary twist, both teams reported their findings to the Neues Allgemeines Journal der Chemie in the same year, each confirming the presence of a new “earth.”
A mild naming dispute followed. Klaproth suggested “ochroite earth,” while Berzelius and Hisinger proposed “ceria,” honoring the dwarf planet Ceres, discovered just two years earlier. Ceria ultimately won out, largely thanks to Hisinger’s status as a respected industrialist and science patron. Although Hisinger brought prestige to the discovery, it was likely the young Berzelius who performed most of the analysis.
The two teams differed in interpreting their findings. Klaproth believed the “earth” to be an element in its own right—a common misconception at the time, as seen with yttria. However, Berzelius, demonstrating insight that would later cement his status as one of chemistry’s great pioneers, correctly suspected that ceria was an oxide of a new element. This was just the beginning for both scientists: Berzelius would go on to revolutionize chemistry by helping to formalize atomic theory, establishing precise atomic mass calculations, and creating the modern system of chemical symbols. Klaproth, already famous for discovering uranium and zirconium, continued his own distinguished career, studying elements such as strontium, titanium, and tellurium.
The parallel discoveries of yttria and ceria revealed a trend that would become familiar: rare earths rarely came alone, and understanding them required scientific acumen as well as international collaboration.
The Plot Thickens: Mosander’s Quest to Untangle Rare Earths #
The discoveries of yttria and ceria had opened Pandora’s box, but science wasn’t yet ready to look inside—not for another two decades. As chemistry advanced, scientists began to understand that their initial assumptions needed revision: these “earths” weren’t pure elements, but metal oxides. Ceria was reclassified as cerium oxide, and yttria as yttrium oxide. This seemed straightforward enough—until the calculations began.
Scientists encountered a recurring issue: when they attempted to determine the atomic masses of these “pure” elements, the results varied across different samples, suggesting inconsistencies that defied explanation. It became clear that these so-called pure elements might actually be mixtures.
Enter Carl Gustaf Mosander, assistant to Jöns Jacob Berzelius. Beginning in the 1820s, Mosander launched what would become a two-decade investigation into these enigmatic oxides. He began with cerium, subjecting it to countless reactions in pursuit of its true composition. In one experiment, he heated cerium oxide with sodium and chlorine, isolating metallic cerium—but he also observed something unusual. Cerium compounds varied in color and density depending on their mineral source, suggesting that “cerium oxide” might conceal additional elements.
Throughout his work, Mosander’s colleague Friedrich Wöhler urged him to publish his findings. However, Mosander was meticulously cautious—perhaps too cautious for Wöhler’s taste. Mosander refused to make any announcements until he was absolutely certain of his results, a thoroughness that, while frustrating to colleagues, would ultimately lead to groundbreaking discoveries.
Hidden Elements: When Two Became Six #
Mosander’s painstaking patience finally paid off in 1839. After years of meticulous investigation, he confirmed his suspicion that cerium oxide contained more than just cerium. Within it, he identified a new element, which he named “lanthanum”—from the Greek for “to escape notice.” The name couldn’t have been more fitting for an element that had remained hidden in plain sight for so long.8
But cerium oxide held further surprises. In 1841, Mosander’s experiments uncovered yet another element. He named it “didymium”—from the Greek word for “twin”—because it seemed to be chemically similar to lanthanum. Though his colleagues, including Wöhler and Berzelius, found the name unusual, Mosander’s methods were reshaping rare earth chemistry. (Interestingly, didymium would later prove to be a mixture itself, ultimately yielding the elements praseodymium and neodymium in the 1880s.)
Mosander’s work was pioneering for its time. He developed advanced techniques for repeated crystallization and gravimetric analysis—methods requiring extreme precision and patience. In 1843, Berzelius documented these techniques, detailing how Mosander had separated cerium, lanthanum, and didymium from what had initially appeared to be a single substance.
Building on these breakthroughs, Mosander turned his attention to yttrium oxide, analyzing gadolinite with his refined methods. In 1843, he successfully isolated two additional elements: “erbium” and “terbium,” leaving “yttrium” for the remaining fraction. This work showcased Mosander’s advanced approach, utilizing oxalate precipitation to separate the elements based on their reactivity and unique properties in color and crystal form.
What had started as two mysterious “earths”—cerium and yttrium—had now revealed themselves as six distinct elements. Mosander’s work hadn’t just expanded the periodic table; it had demonstrated that rare earths were far more complex than anyone had imagined.
A New Era: When Light Revealed Chemical Secrets #
After Mosander’s revelation that two rare earths could conceal six distinct elements, chemistry faced a new challenge. His separation methods, though groundbreaking, were exceedingly time-consuming, often requiring months or even years of repeated crystallizations and precise measurements. As organic chemistry advanced rapidly with its discoveries, research on rare earths slowed significantly.
Then, in 1859, everything changed. Robert Bunsen and Gustav Kirchhoff introduced a groundbreaking technique: spectral analysis. Instead of labor-intensive separations, chemists could now identify elements by the unique colors of light they emitted when heated—like a chemical “fingerprint” displayed as spectral lines. Each element had its distinctive spectral pattern, making identification faster, more accurate, and far less labor-intensive than before.
This innovation arrived just as Dmitri Mendeleev and Julius Lothar Meyer were independently developing the periodic table in the late 1860s. Their table systematically organized elements by atomic weights and properties, allowing chemists to see relationships between elements and even predict the existence and properties of unknown ones. For the first time, chemistry had a framework that didn’t just catalog elements but illuminated the structure of matter itself.
The combination of spectral analysis and the periodic table gave new fuel to the interest in rare earth elements. The table suggested gaps where new elements might exist, while spectral analysis provided a powerful tool for identifying them. What had begun with Mosander’s patient crystallizations could now be pursued with the precision of light, setting the stage for a new wave of discoveries that would further unlock the rare earth puzzle.
Masters of Separation: The Next Generation of Rare Earth Chemists #
The advances in spectral analysis and the organizational power of the periodic table attracted fresh minds to the rare earth puzzle. Among these new investigators was Swiss chemist Jean Charles Galissard de Marignac, who recognized a critical need for precise atomic weight measurements to better understand these elusive elements. Building on Mosander’s methods, Marignac recalculated the atomic weights of cerium, lanthanum, and didymium with extreme precision. His work came at a crucial time: following the 1860 Karlsruhe Congress, where the need for standardized atomic weights was discussed, Marignac’s careful measurements helped unite chemists across Europe around consistent values.
Another Swiss chemist, Marc Delafontaine, turned his focus to Mosander’s enigmatic discoveries, particularly terbium. Though several chemists had worked to isolate terbium, making it difficult to attribute its discovery to one individual, Delafontaine’s systematic studies clarified its properties. Yet his most intriguing work centered on didymium—the “twin” element Mosander had identified. Delafontaine began to suspect didymium was not a single element, observing subtle differences in its spectral lines that hinted at greater complexity.
French chemist Paul Émile Lecoq de Boisbaudran would later confirm Delafontaine’s suspicion. Working with samarskite, a mineral rich in rare earths, Lecoq de Boisbaudran used spectral analysis to reveal that didymium was not an individual element but a mixture. In time, this “twin” element would split into two distinct elements, praseodymium and neodymium. Once again, rare earth chemistry demonstrated that what seemed straightforward often concealed layers of complexity.
From “Twin” Elements to Modern Technology: The Final Discoveries #
The rare earth puzzle was far from complete in 1885 when Austrian chemist Carl Auer von Welsbach decided to take another look at didymium. Working with gadolinite, he achieved what others couldn’t: separating didymium into two distinct elements. One produced green oxides, which he named praseodidymium (“green twin”), and the other gave pink ones, which became neodidymium (“new twin”)—the elements now known as praseodymium and neodymium.
Von Welsbach wasn’t just interested in theoretical discovery—he saw practical potential in these elements. His most successful invention was the incandescent gas mantle, which used rare earth salts, particularly thorium and cerium, to produce bright and efficient light. This technology proved so valuable that it helped establish the lighting company Osram and made von Welsbach financially successful—a rare case where pure research translated directly into commercial innovation.
The field continued to advance. In 1896, Eugène-Anatole Demarçay identified europium hiding in samarium samples and named it after Europe. Then, as the 19th century drew to a close, both von Welsbach and French chemist Georges Urbain independently discovered that ytterbium contained two distinct elements. Their naming dispute—Urbain’s neoytterbium and lutetium versus von Welsbach’s aldebaranium and cassiopeium—would echo through chemistry for decades. In Germany, “cassiopeium” was used in place of lutetium right up until World War II.
One puzzle piece was still missing: element 61. The gap remained until 1947, when Manhattan Project scientists isolated traces of this elusive element in uranium fission products. They named it promethium, after the Titan who brought fire to humanity—a fitting choice for an element discovered through nuclear research. Unlike its rare earth cousins, promethium is highly radioactive and appears in nature only in trace amounts.
With promethium’s discovery, a chapter in the history of rare earths closed. What began in the 1780s with two mysterious “earths” had revealed itself as a family of 17 elements, each with unique properties and applications. From the early puzzles of obscure oxides to becoming crucial components of modern technology, the story of rare earths stands as a reminder that scientific progress often comes not in dramatic leaps, but through generations of patient, persistent work.
Critical Metals Handbook, 1st ed.; Gunn, G., Ed.; John Wiley & Sons, 2013. https://doi.org/10.1002/9781118755341. ↩︎
Höppe, H. Rare-Earth Elements: Solid State Materials: Chemical, Optical and Magnetic Properties; De Gruyter Graduate; De Gruyter: Berlin, DE, 2024. https://doi.org/10.1515/9783110680829. ↩︎
Voncken, J. H. L. The Rare Earth Elements; SpringerBriefs in Earth Sciences; Springer: Cham, CH, 2016. https://doi.org/10.1007/978-3-319-26809-5. ↩︎
Gschneidner, K. A.; Eyring, L. Handbook on the Physics and Chemistry of Rare Earths: Two-Hundred-Year Impact of Rare Earths on Science; Handbook on the Physics and Chemistry of Rare Earths; Elsevier Science: Amsterdam, NL, 1988; Vol. 11. ↩︎ ↩︎
Rowlatt, J. Rare Earths: Neither Rare, nor Earths. BBC News. March 23, 2014. https://www.bbc.com/news/magazine-26687605 (accessed 2024-08-18). ↩︎
Klinger, J. M. A Historical Geography of Rare Earth Elements: From Discovery to the Atomic Age. Extr. Ind. Soc. 2015, 2 (3), 572–580. https://doi.org/10.1016/j.exis.2015.05.006. ↩︎
Not to be confused with Svante Arrhenius, the Swedish Nobel Prize winner who was born in 1859. ↩︎
Episodes from the History of the Rare Earth Elements; Evans, C. H., Ed.; Springer Netherlands: Dordrecht, NL, 1996. https://doi.org/10.1007/978-94-009-0287-9. ↩︎