The Science of Glass: From Structure to Stability
Table of Contents
Glass is a unique material, defined by its disordered atomic structure and metastable state. Unlike crystalline solids, which have an orderly arrangement of atoms, glass exists in an amorphous form, constantly seeking but never reaching equilibrium.
The glass transition temperature (\(T_g\)) marks the point where glass softens and transitions from a rigid solid to a more fluid state, while the behavior of supercooled liquids and structural relaxation explains how glass forms and retains its properties. Through these processes, glass blurs the line between solid and liquid, making it one of the most versatile and scientifically intriguing materials.
Glass is one of the most versatile materials in the world, found in everything from windows and containers to fiber optics and advanced technologies. Despite its everyday use, glass has a unique atomic structure that distinguishes it from other materials.1
Unlike crystalline solids, glass has a disordered arrangement of atoms. This disordered structure results in properties that make glass distinct. Even though it appears solid, glass exists in a non-equilibrium state, meaning that its atomic arrangement is constantly shifting toward a liquid phase, although over extremely long timescales.
So, what exactly sets glass apart from other solids, and how does its structure influence its behavior? Understanding these questions reveals the scientific significance of glass and its long-lasting technological role.
The Foundation of Solid Matter #
To understand glass, it’s important to first understand what defines a solid.
Solids are one of the primary states of matter. Unlike liquids and gases, which flow and change shape, solids maintain their form and volume without needing a container. This stability comes from the way their atoms are arranged and bonded together.
At the atomic level, solids can be categorized into two types: crystalline and amorphous. In crystalline solids—such as diamonds or metals—atoms are arranged in a highly ordered, repeating pattern. This regular structure gives crystalline materials their rigidity and strength. In contrast, amorphous solids, like glass, lack this ordered arrangement. Instead, their atoms are disorganized, resembling the structure of a liquid that has been rapidly cooled and “frozen” in place.2 3
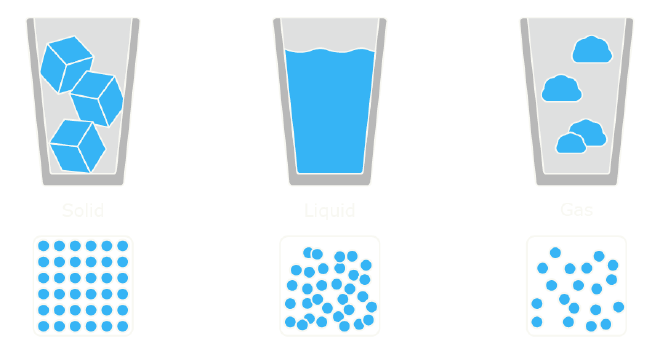
This disordered structure is what makes glass unique. It behaves like a solid in the short term, but over long periods, it exhibits subtle, almost imperceptible movement. This is due to the fact that glass never truly reaches a state of thermodynamic equilibrium. Its atomic structure is always in a slow process of shifting, inching toward a liquid-like state.
Deforming any solid, including glass, requires applying an external force that exceeds the material’s yield stress—the point at which the material starts to change shape. For glass, this threshold varies with temperature: higher temperatures reduce the force needed for deformation. However, even at room temperature, glass exists in this delicate state between solid and liquid, which is one of the reasons it has such unusual and fascinating properties.
So, what exactly sets glass apart from other materials, and how does its disordered atomic structure shape its behavior? In the next section, we’ll explore these questions and see how glass blurs the line between solid and liquid.
The Structural Divide: What Makes Glass Unique #
The distinct atomic structure of glass is what sets it apart from other materials.
In crystalline solids, atoms are arranged in a highly ordered and repeating pattern. This arrangement is known as long-range translational symmetry. It gives crystalline materials predictable properties, such as sharp melting points and anisotropy—meaning their mechanical properties, like strength or elasticity, vary depending on the direction of force applied.
Glass, however, is different. It is an amorphous solid, meaning its atoms are arranged in a disordered way. This lack of regular atomic structure results in isotropic behavior, where its properties are the same in all directions. For example, glass is equally strong regardless of the direction you apply force. Because glass lacks the strict order of a crystal, it also doesn’t have a defined melting point. Instead, it softens gradually as temperature rises, transitioning smoothly from a rigid state to a more fluid one.
A classic example of this structural divide is silica (\( \ce{SiO2} \)). In its crystalline form, it becomes quartz, which has a well-defined melting point and anisotropic properties. In its amorphous form, as glass, silica behaves differently, exhibiting the gradual softening and isotropic properties characteristic of glasses.
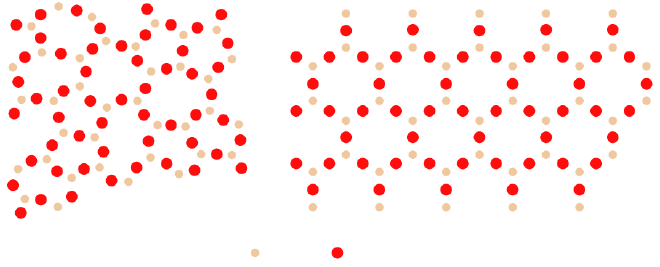
Interestingly, glass isn’t limited to materials like silica. Many other substances—including metals and polymers—can also form amorphous, glassy structures. This happens when they are cooled rapidly enough to avoid crystallization. Despite their chemical differences, all these materials share one key feature: the disordered, non-crystalline atomic structure that defines glass.
In essence, the fundamental distinction between crystals and glass comes down to atomic order: where crystals rely on structured precision, glass embraces atomic disorder.
The Metastable Nature of Glass #
One of the most distinctive features of glass is that it exists in a metastable state—a non-equilibrium condition that sets it apart from most materials.
Typically, when materials cool, their atoms arrange themselves into a stable, ordered structure, forming a crystalline solid. This structure is thermodynamically favorable because it represents the lowest energy state for the material. However, glass breaks this rule. Even though it would be more stable as a crystal, glass remains in an amorphous state, where its atoms are disordered.4 5
This disordered structure is what makes glass metastable. It’s not in full equilibrium, but it can stay in this state for an extremely long time without changing. Unlike crystalline solids, which have a precise arrangement of atoms, glass lacks this regularity. As a result, it has several distinct thermodynamic properties. For example:
- Glass has a larger molar volume because its atoms aren’t packed as tightly as in crystals.
- It has higher entropy (a measure of disorder) due to the many possible ways its atoms can be arranged.
- Glass also has higher enthalpy and Gibbs free energy, meaning it holds more energy than a corresponding crystalline solid.
These characteristics indicate that glass is less stable than a crystal, but because it cools so rapidly during its formation, it gets “trapped” in this non-equilibrium state. This rapid cooling prevents the atoms from organizing into a more stable crystalline structure.
Even though glass would naturally prefer to form a crystal, it remains amorphous indefinitely because the energy needed to overcome its metastable state and trigger crystallization is too high under normal conditions. This balance—disordered, yet stable enough to persist—is what defines the unique nature of glass.
In short, glass occupies a middle ground: stable enough to last for centuries, yet constantly in a subtle, slow-moving journey toward equilibrium.
The Glass Transition: From Solid to Liquid-Like Behavior #
One of the defining features of glass is the glass transition, a process that distinguishes it from other amorphous materials.3
The glass transition describes the gradual shift from a rigid, solid state to a more viscous, rubbery state as temperature increases. This transformation occurs over a temperature range and is marked by the glass transition temperature (\(T_g\)). As the temperature rises, glass does not melt like a crystalline solid. Instead, it softens, becoming less brittle and more flexible.
At the glass transition temperature, the atomic arrangement of the material becomes essentially fixed, locking the glass into its amorphous state. When the material is cooled rapidly enough, preventing crystallization, it undergoes a process called vitrification, where it transitions into a solid glass from a liquid.
The glass transition temperature isn’t a sharp, single point, but rather a range that depends on the material’s thermal history. Factors like the cooling rate can influence where this transition occurs. Faster cooling typically results in a higher \(T_g\), as the atoms have less time to settle into a more stable arrangement. Slower cooling allows more atomic movement, lowering the \(T_g\). As glass approaches its \(T_g\), its viscosity increases dramatically, meaning it becomes harder for its atoms to move and rearrange.
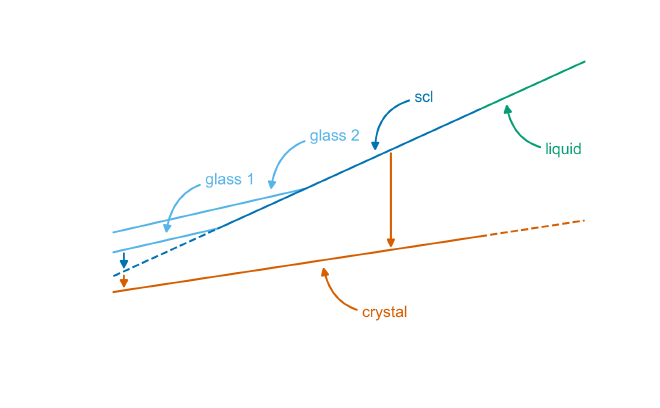
This behavior is fundamentally different from crystalline solids, which melt at a defined melting point (\(T_m\)). In glass, the transition from a liquid to a solid is more gradual, and the properties of the material during this range are influenced by both temperature and time.
Understanding the glass transition is important because it directly affects how glass performs in various applications. The temperature range at which glass softens and solidifies impacts its durability, stability, and usability in everything from everyday objects to advanced technologies.
The Dynamics of Supercooled Liquids: The Path to Glass Formation #
At the heart of glass formation is the behavior of supercooled liquids—liquids cooled below their freezing point without crystallizing.
As a supercooled liquid approaches the glass transition, its atoms continue to shift through a process called structural relaxation. Even when thermal equilibrium is reached, this internal restructuring persists, although more slowly as the temperature drops. This process is central to understanding how glass forms and maintains its unique properties over time.
The relaxation time (\( \tau_{s} \))—the time it takes for the atomic structure to adjust to temperature changes—increases significantly as the liquid cools. When the cooling rate is faster than the material’s ability to relax, the atoms freeze into a disordered arrangement, creating the glassy, non-equilibrium state. This point is marked by the fictive temperature, which indicates when the material transitions from equilibrium to a frozen, non-equilibrium structure.
For many materials, the fictive temperature aligns with the glass transition temperature (\(T_g\)), where the viscosity reaches a level that essentially halts atomic movement, and the material becomes glass.
Conclusion #
The formation and properties of glass are rooted in a complex balance between atomic disorder, temperature, and time. From its metastable nature to the dynamics of supercooled liquids, glass is a material that exists between solid and liquid, equilibrium and non-equilibrium. Its unique atomic structure, combined with the processes that govern its formation, ensures that glass remains one of the most scientifically intriguing and versatile materials in the world.
Mysen, B.; Richet, P. Silicate Glasses and Melts, 2nd ed.; Elsevier: Amsterdam, NL, 2019. https://doi.org/10.1016/C2018-0-00864-6. ↩︎
Vogel, W. Glass Chemistry; Springer: Berlin, DE, 1994. https://doi.org/10.1007/978-3-642-78723-2. ↩︎
Schmelzer, J. W. P.; Gutzow, I. S. Glasses and the Glass Transition; John Wiley & Sons: Weinheim, DE, 2011. https://doi.org/10.1002/9783527636532. ↩︎ ↩︎
Zanotto, E. D.; Mauro, J. C. The Glassy State of Matter: Its Definition and Ultimate Fate. J. Non-Cryst. Solids 2017, 471, 490—495. https://doi.org/10.1016/j.jnoncrysol.2017.05.019. ↩︎
Montazerian, M.; Zanotto, E. D. The Glassy State. In Encyclopedia of Materials: Technical Ceramics and Glasses; Elsevier, 2021; Vol. 2, pp 448—461. https://doi.org/10.1016/B978-0-12-803581-8.11728-X. ↩︎